Flu A/B & COVID-19 Ag Combo Rapid Test
3 in 1 COVID-19/Flu A/Flu B Antigen Combo Rapid Test
Intented Use
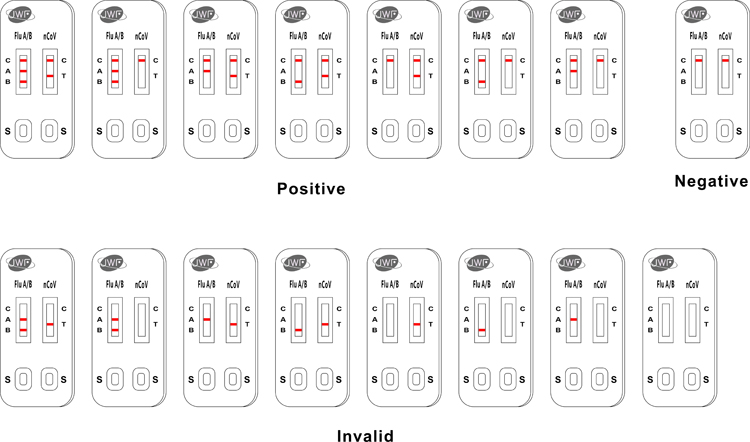
The Jinwofu® COVID/Flu Ag Combo Test is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 and Influenza A and Influenza B present in human nasopharyngeal specimens.
The Jinwofu® COVID/Flu Ag Combo Test for rapid detection of SARS-CoV-2, Influenza A and Influenza B is a differentiated test, such that SARS-CoV-2 viral antigens can be distingushed from Influenza A or Influenza B viral antigens from a single specimen using a single device.
Advantage
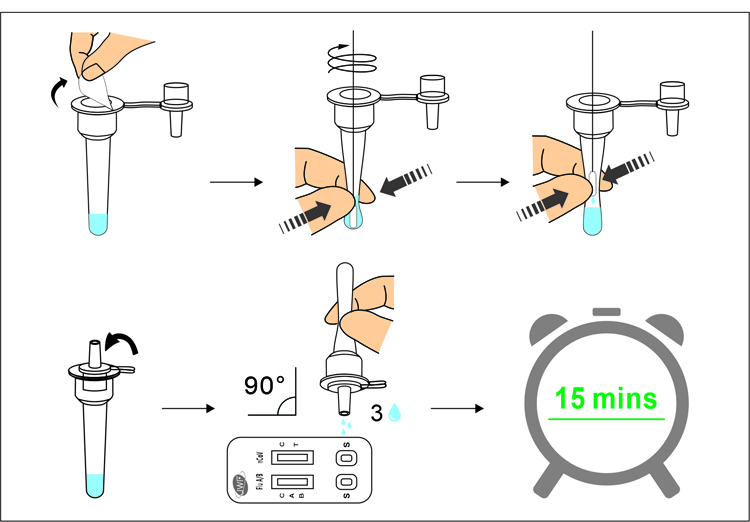
- Getting results in 15 ~ 30 minutes
- Easy and convenient testing process for professionals
- One-time specimen collection for both COVID-19 and Influenza testing
- Simultaneous detection of COVID-19 and Influenza antigens
- Time savings, material conservation, and patient comfort testing.
- Suitable for Point of Care Testin