Monkeypox virus Antigen Test Kit

The monkeypox virus is an orthopoxvirus that causes mpox (monkeypox), a disease with symptoms similar to smallpox, although less severe. While smallpox was eradicated in 1980, mpox continues to occur in countries of central and west Africa. Since May 2022, cases have also been reported from countries without previously documented mpox transmission outside the African region. Two distinct clades of the monkeypox virus have been identified: Clade I (previously known as the Congo Basin (central African) clade and Clade II (the former west African clade).
Mpox is a zoonosis, a disease that is transmitted from animals to humans, with cases often found close to tropical rainforests where there are animals that carry the virus. Evidence of monkeypox virus infection has been found in animals including squirrels, Gambian pouched rats, dormice, different species of monkeys and others.
The disease can also spread from humans to humans. It can be transmitted through contact with bodily fluids, lesions on the skin or on internal mucosal surfaces, such as in the mouth or throat, respiratory droplets and contaminated objects.
Description
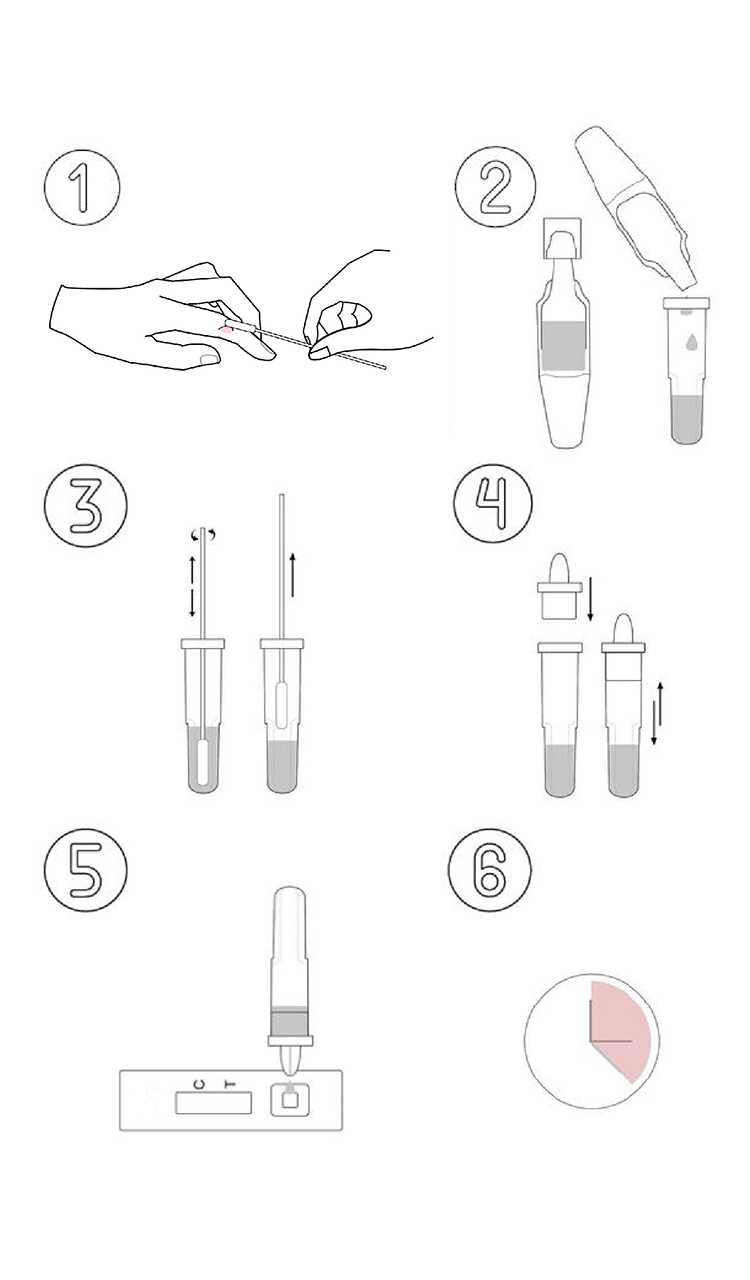
Jinwofu's innovative Monkeypox Antigen Rapid Test Kit is lateral flow immunoassays in cassette format for the qualitative detection of monkeypox antigen during infection.
The most common symptom of monkeypox virus infection is skin lesions. While infected by monkeypox virus, the virus antigen can be detected on the skin lesion exudate, acne scab swabs, or nasal swabs. The Jinwofu monkey pox antigen rapid test cassette comes with a thin swab so that healthcare professionals can easily collect test samples from a patient’s skin lesions.
Features
- Rapid detection of monkeypox (Mpox) virus antigen
- 15-minute rapid test cassette
- Easy-to-operate
- CE-IVD certified
- Available in 25 tests/box
Monkeypox Antigen Rapid Test Procedure